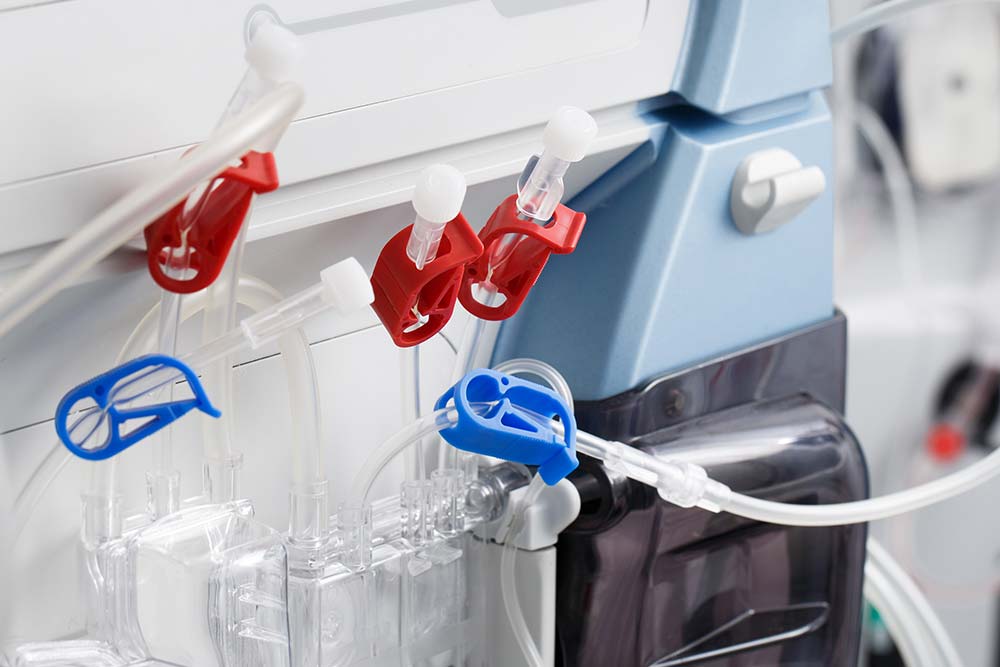
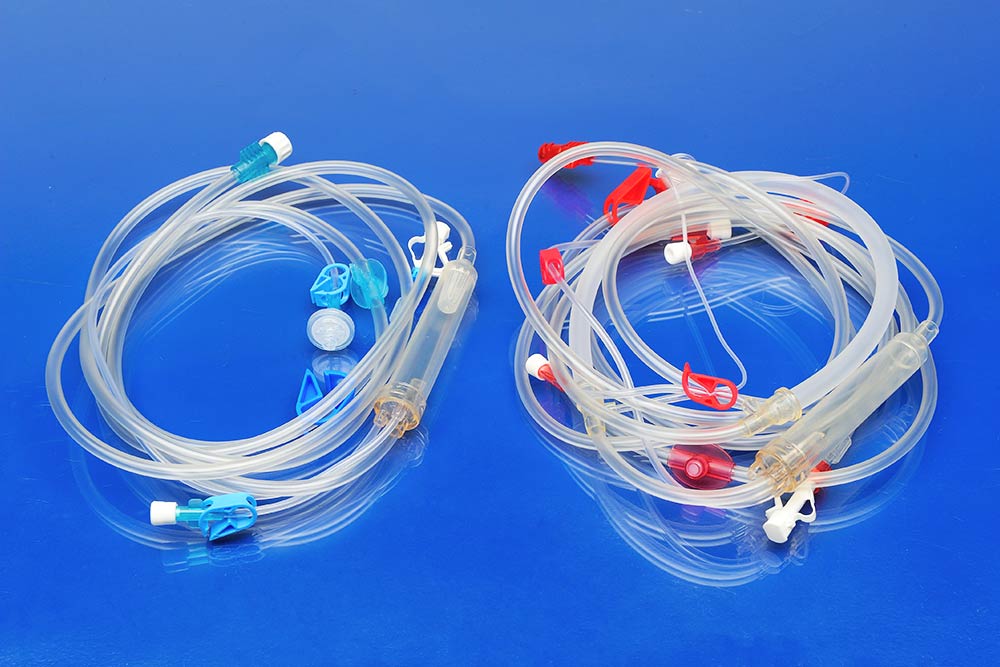
Hemodialysis compound has passed the USP biocompatibility test. The raw materials are carefully chosen and checked. The process control is quite strict. It is suitable for injection and extrusion with good processing stability. We have a complete range of specifications. It is used for products such as tubes and components of disposable hemodialysis bloodline.
Characteristics
- It passes the USP or ISO 10993 biocompatibility test.
- The flexible compound has high transparency, good elasticity. And the rigid compound has high toughness.
- We can cooperate with the development of Phthalate free formula.
- It obtains the US FDA DMF registration (No. 26526).
- It is suitable for EO sterilized medical devices.(it can be specially developed if steam sterilization is required)
Applications
- Dialysis main tube, pump tube, monitoring tube
- Drip chamber, cover of drip chamber
- T connector, dialysis connector, sampling connector
- Male luer, female luer, rotary joint, pressure monitoring protective cover
- A.V.F needle tube
Specifications
| Spec. | Application | HardnessASTM D2240 | Specific GravityASTM D792(g⁄cm3) | Tensile StrengthASTM D638(kgf⁄cm2) | ElongationASTM D638(%) | Data Sheet |
|---|